 | 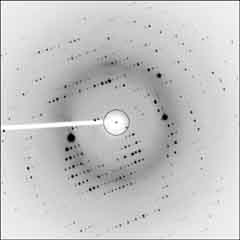 |
| Needle crystals (hexagonal) of TcGS grown from aqueous
iso-propanol, and diffraction image. (structure not determined) | |
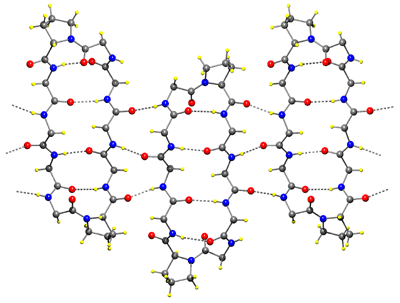
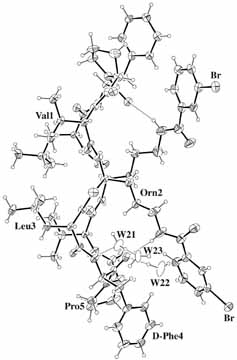
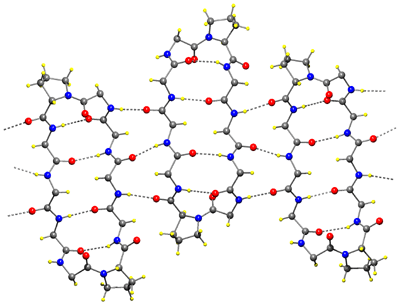
BrGS: cyclo(-Val-Orn(BrBz)-Leu-D-Phe-Pro-)2 TcGS: cyclo(-Val-Orn(Tc)-Leu-D-Phe-Pro-)2 [BrBz=m-bromobenzoicyl, Tc=trichloroacetyl] |
To display this figure in your brawser, a tool, Chime, is necessary. Click buttons to show different views, and molecule will be rotated by your mouse. Shift and click = zoom; Option and click = translate |
 |  |
| Needle crystals (hexagonal) of TcGS grown from aqueous
iso-propanol, and diffraction image. (structure not determined) | |
 |  |
 | 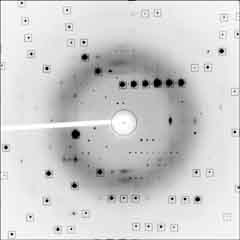 |
| Plate crystals (monoclinic) of TcGS grown from aqueous DMF, and diffraction image. (structure solved) Weak satelite spots are recorded at low resolution. It is postulated that these cell are close to hexagonal cell. | |
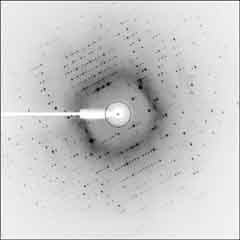 |
Left: Diffraction image of AcGS, which is similar to that of needle crystalss of TcGS. This is a typical one for hexagonal crystals of AcGS. (structure not determined) P6122 or P6522 a=b=28.3 A c=54.9 A alpha=beta=90 deg. gamma=120 deg. V=36,000 A3 Schmidt, G. M. J., Hodgkin, D. C., and Oughton, B. M. (1957). Biochem. J. 65, 744-751. Oh! My birth year! |
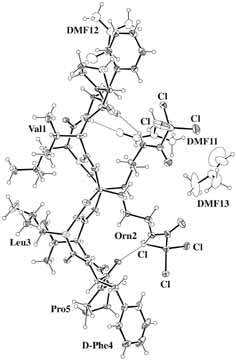
| Get PDB coordinates: |  TcGS TcGS |
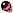 BrGS BrGS |
 Back to the structure index
Back to the structure index