|
3-Methoxysulfoxide-(2R,3R)-threoninyl
desoxazoline-ascidiacyclamide
cyclo(-Ile-D-allo-MsThr-D-Val-Thz-Ile-D-allo-MsThr-D-Val-Thz-) [Thz=thiazole, Ms=-0SO2OMe, Thr=(2S,3R), D-alloThr=(2R,3R)] 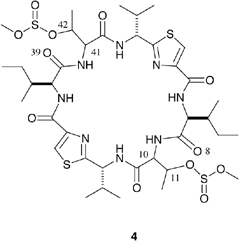
| 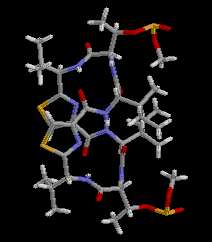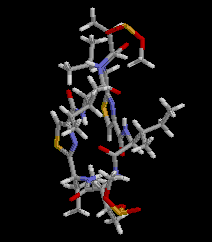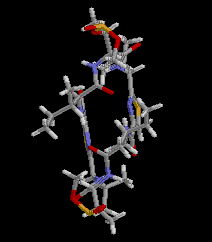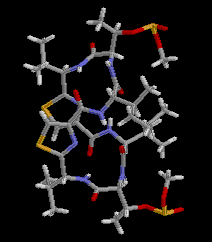 RasMol2.7.2.1, and stucked by GifBuilder (Yves Piguet, 1997) |
Reference: Copyright © International Union of Crystallography
Doi, M., Asano, A., Usami, Y., Katsuya, Y., Nakai, M., Sasaki, M., Taniguchi, T. and Hasegawa, H. (2001). A folded conformation of an ascidiacyclamide derivative: 3-methoxysulfoxide-(2R,3R)-threoninyl desoxazoline-ascidiacyclamide. Acta Crystallogr. E57, o1019-o1021.
See also Index of Section E.
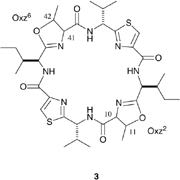 Ascidiacyclamide (ASC) (3) is
a symmetric cyclic peptide (Fig.1) containing unusual amino acids such as
oxazoline (Oxz) and thiazole (Thz). Two major conformations and their
conformational equilibrium have been suggested for ASC. In our series of
studies, the relationships between ASC conformation and symmetry of the
chemical structure have been examined; additionally we have studied the
asymmetric modifications which disturb the C2-symmetry of ASC and how this
affects the ASC structures in both the solid state and in solution. Here, we
extend the asymmetric modifications by substituting the Oxz residues with
their diastereomers.
Ascidiacyclamide (ASC) (3) is
a symmetric cyclic peptide (Fig.1) containing unusual amino acids such as
oxazoline (Oxz) and thiazole (Thz). Two major conformations and their
conformational equilibrium have been suggested for ASC. In our series of
studies, the relationships between ASC conformation and symmetry of the
chemical structure have been examined; additionally we have studied the
asymmetric modifications which disturb the C2-symmetry of ASC and how this
affects the ASC structures in both the solid state and in solution. Here, we
extend the asymmetric modifications by substituting the Oxz residues with
their diastereomers.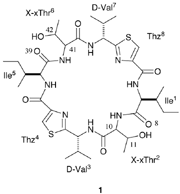
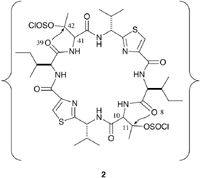
ASC is synthesized from a cyclic hexapeptide, (1), using thionyl chloride , as shown in Fig.1. Oxz rings are formed from the threonine residues via the chlorosulfoxide intermediate (2) after reacting for 1-2 days at 0-4 C. For synthesis of the ASC diastereomers, the threonine residues of (1) are replaced by the following threonine diastereomers: Thr, allo-threonine (aThr), D-Thr and D-aThr. A total of ten diastereomers of (1) were synthesized and their configurations at positions (10,11,41,42) are (S,S,S,S), (S,S,S,R), (S,S,R,R) , (S,S,R,S), (S,R,S,R), (S,R,R,R), (S,R,R,S), (R,R,R,R), (R,R,R,S) and (R,S,R,S). Natural ASC is synthesized from (S,S,S,S)-(1).
 For seven of the ten
diastereomers, the reaction of Oxz formation was completed under the reported
conditions. However, this reaction did not complete or delayed for the three
diastereomers having (R,R,R,R), (R,R,R,S) and (R,S,R,S) configurations. These
configurations imply that residues 2 and 6 are D-amino acids (Ca at positions
10 and 41). We presume that a particular conformation, which hinders the
completion of the reaction, is present in the intermediates (2) derived from
these three diastereomers. Such a conformation is interesting because it is
stable, even in thionyl chloride solution. To clarify this conformation, three
methyl sulfoxide derivatives of 4 were isolated, by the addition of methanol
to intermediates (2), and the structure of (R,R,R,R)-(4) was analyzed.
For seven of the ten
diastereomers, the reaction of Oxz formation was completed under the reported
conditions. However, this reaction did not complete or delayed for the three
diastereomers having (R,R,R,R), (R,R,R,S) and (R,S,R,S) configurations. These
configurations imply that residues 2 and 6 are D-amino acids (Ca at positions
10 and 41). We presume that a particular conformation, which hinders the
completion of the reaction, is present in the intermediates (2) derived from
these three diastereomers. Such a conformation is interesting because it is
stable, even in thionyl chloride solution. To clarify this conformation, three
methyl sulfoxide derivatives of 4 were isolated, by the addition of methanol
to intermediates (2), and the structure of (R,R,R,R)-(4) was analyzed.
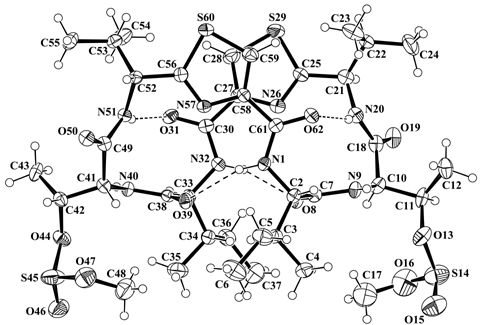
PLATON (Spek,1998) drawing
X-Ray Data Summary
| formula | C38 H60 N8 O12 S4 | ||
| weight | 949.18 | ||
| symmetry | orthorhombic | ||
| space group | P212121 | ||
| Cell | Crystal | ||
| a (Ang) | 10.7820(3) | description | block |
| b (Ang) | 19.6026(4) | colour | colorless |
| c (Ang) | 22.5181(7) | size (mm3) | 0.08x0.08x0.08 |
| alpha (deg) | 90.0 | Dx (g/ml) | 1.325 |
| beta (deg) | 90.0 | F(000) | 2016 |
| gamma (deg) | 90.0 | mu (mm-1) | 0.265 |
| V (Ang^3) | 4759.3(2) | ||
| Z | 4 | ||
| Refinement | Diffrn measurement | ||
| Flack | 0.38(15) | device type | Rigaku RAXIS-IV |
| parameters | 560 | decay | --- |
| restraints | 0 | index limit | -13-h-13,-23-k-23,0-L-27 |
| R_factor_all | 0.0783 | Rint | 0.0554 |
| R_factor_gt | 0.0750 | reflections(meas) | 4973 merged form 16269 |
| wR_factor_ref | 0.2592 | reflections(merged) | 4759 .gt. 2sigma(I) |
| wR_factor_gt | 0.2583 | Structure | |
| shift/su_max | 0.042 | solution | SHELXS-97 |
| shift/su_mean | 0.004 | refinement | SHELXL-97 |
Get PDB coordinates
 Back to the structure index
Back to the structure index
Date: Nov. 2001