| Desoxazoline ascidiacyclamide dimer
monohydrate, 3DMF solvated cyclo(-Ile-Thr-D-Val-Thz-Ile-D-alloThr-D-Val-Thz-)2 [Thz=thiazole, Thr=(2S,3R), D-alloThr=(2R,3R)] 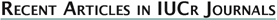 This structure was reported in "Recent Artricles in IUCr Journals" of IUCr Newletter Volume 10 Number 3 (2003). Click title, download an extracted part of PDF (2.7MB). The original PDF is distributed, but its size is 21MB. Reference: Copyright © International Union of Crystallography A. Asano, T. Taniguchi, M. Sasaki, H. Hasegawa, Y. Katsuya and M. Doi (2001). A [beta]-sheet structure formed by C--H...O hydrogen bonds between the thiazole rings and amide bonds of a dimeric desoxazoline ascidiacyclamide analogue Acta Crystallographica Section E, Volume 57, Part 09, pages o834---o838. See also Index of Section E. X-Ray diffraction data were measured on SPring-8/BL24XU-A with the approval from Hyogo Prefecture and Japan Synchrotron Radiation Research Institute (C00A24XU-5003N). | 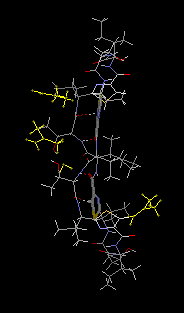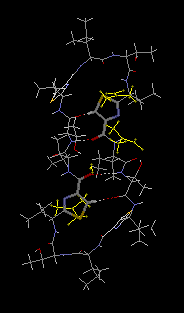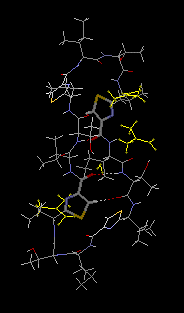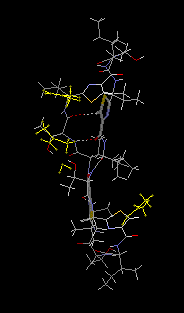 RasMol2.7.2.1, and stucked by GifBuilder (Yves Piguet, 1997) |
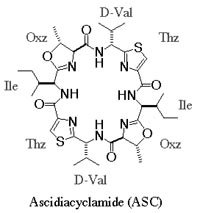
Ascidiacyclamide (ASC) is a cytotoxic peptide isolated from marine organs, and include unusual amino acids, thiazole (Thz) and oxazoline (Oxz). Two major structures are known for this peptide. We have attempted to control its structure by chemical modification, eg. substitution of amino acids or changing chirality. The conformations of ASC analogues seems to be restricted by the rigid blocks composed of Thz and Oxz, because these blocks limit the rotations of peptide backbones (N-Ca and Ca-C bonds). Therefore, we tried to break the Oxz blocks to increase backbone flexibility. The Oxz-block is synthesized from threonine, and such modifications result threonine residues at the Oxz-blocks. Threonine has two chiral carbons at alpha and beta-positions, and both carbons could be targets of the chiral modifications (a diastereomer of threonine at C\b position is called as allo-threonine, aThr). Such desoxazoline-ASC analogues (dASC) rather converged to a single conformation of a folded-form. It was postulated that a molecular size of these cyclic peptides (24-membered ring) could limit conformational variations.
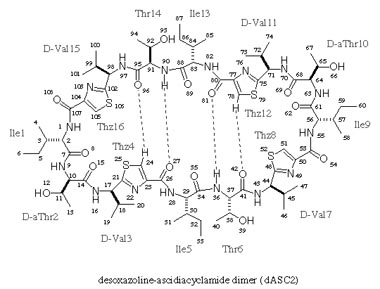
Thus, we attempted a dimerization of these dASC diastereomers to give more conformational freedom to the peptide backbone. The dimeric desoxazoline-ASC (dASC2) analogues have a 48-membered ring for a 24- membered ring of ASC and dASC analogues. Crystals of [Thr,D-aThr]-dASC2 was obtained from dASC2 diastereomers. The structure of this analogue is completely different from folded or squared structure of ASC and a related peptide, patellamide. We report the crystal structure of [D-aThr,Thr]-dASC2.
| Precursive liner octapeptide (6 units) |
cyclization ----> at low concentration |
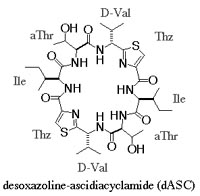 |
| Desoxazoline ascidiacyclamide |
create oxazoline rings ----> SO2Cl |
 |
| Precursive liner octapeptide (6 units) |
cyclization ----> at high concentration |
 |
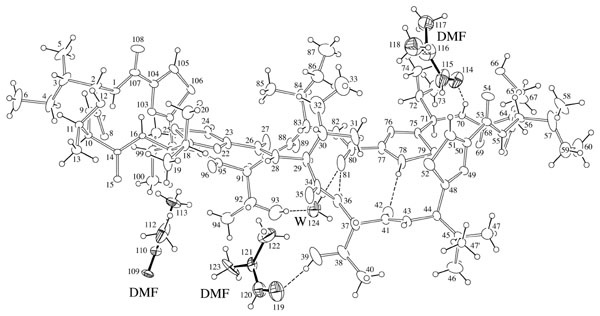
ORTEP-III (Burnett, 1996) drawing
A stereo view is here (44KB).
X-Ray Data Summary
| formula | C72H112N16O16S4 +3(C3H7NO) +H2O | ||
| weight | 1823.32 | ||
| symmetry | monoclinic | ||
| space group | P21 | ||
| Cell | Crystal | ||
| a (Ang) | 11.55630(10) | description | cubic |
| b (Ang) | 9.9957(2) | colour | colorless |
| c (Ang) | 42.9208(10) | size (mm3) | 0.01x0.01x0.01 |
| alpha (deg) | 90.0 | Dx (g/ml) | 1.222 |
| beta (deg) | 91.3492(15) | F(000) | 1956 |
| gamma (deg) | 90.0 | mu (mm-1) | 0.168 |
| V (Ang^3) | 4956.55(16) | ||
| Z | 2 | ||
| Refinement | Diffrn measurement | ||
| Flack | -0.17(14) | device type | Rigaku RAXIS-IV |
| parameters | 1119 | decay | --- |
| restraints | 4 | index limit | -13-h-13,-11-k-12,0-L-51 |
| R_factor_all | 0.0973 | Rint | 0.0378 |
| R_factor_gt | 0.0967 | reflections(meas) | 15629 include Friedel pairs |
| wR_factor_ref | 0.2592 | reflections(merged) | 8890 .gt. 2sigma(I) |
| wR_factor_gt | 0.2583 | Structure | |
| shift/su_max | 0.017 | solution | SHELXS-97 |
| shift/su_mean | 0.001 | refinement | SHELXL-97 |
Get PDB coordinates
 Back to the structure index
Back to the structure index
Date: Aug. 2001