
L-b-threonine lactone
[4(R)-(N-Benzoylamino)-5(R)-methyltetrahydrofuran-2-one]
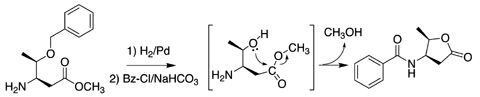
Lactonization spontaneously processed under weak base, and the elongation of backbone makes it easy. @Sep. 2003
 L-b-threonine lactone [4(R)-(N-Benzoylamino)-5(R)-methyltetrahydrofuran-2-one] |

Lactonization spontaneously processed under weak base, and the elongation of backbone makes it easy. @Sep. 2003 |
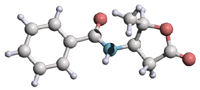 Published: Akiko Asano and Mitsunobu Doi 4(R)-(N-Benzoylamino)-5(R)-methyltetrahydrofuran-2-one: an L-b-threonine analogue. Acta Cryst. E59, o1486-o1487 (2003). Copyright © International Union of Crystallography |
| formula | C12 H13 N O3 | ||
| weight | 219.23 | ||
| symmetry | monoclinic | ||
| space group | P21 | ||
| Cell | Crystal | ||
| a (A) | 8.521(1) | description | needle |
| b (A) | 5.1143(6) | colour | Colorless |
| c (A) | 12.401(2) | size (mm) | 0.5x0.06x0.03 |
| a (deg) | 90 | Dx (g/ml) | 1.364 |
| b (deg) | 99.098(2) | F(000) | 232 |
| g (deg) | 90 deg. | m (mm^-1) | 0.099 |
| volume | 533.6(1) | wavelength (A) | 0.71073 |
| Z | 2 | ||
| Diffrn measurement | Refinementspace | ||
| device type | Bruker AXS SMART APEX | Flack x parameter | -0.06(6) |
| Rint | 0.0236 | parameters | 146 |
| T (K) | 120.0 | restraints | 1 |
| theta max (deg) | 27.86 | R_factor_gt | 0.0434 |
| reflections obs. | 4798 | wR_factor_gt | 0.1156 |
| reflections for ref. | 1339 .gt. 2s(I) | Dr max (e/A^3) | 0.404 |
| Dr min (e/A^3) | -0.155 | ||
| Structure | D/s max | <0.001 | |
| solution | SHELXS-97 | Goodness of fit | 1.121 |
| refinement | SHELXL-97 | fraction for q max | 0.976 |
