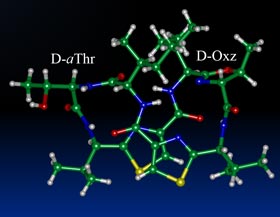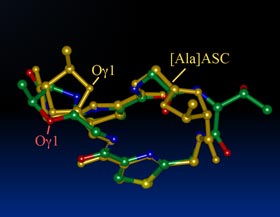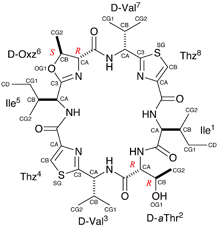
| Synopsis: This compound, two Oxz residues of asciduacyclamide were replaced with D-aThr and (2R,3S)-Oxz (D-Oxz). The overall structure is similar to the folded from of ASC analogues previously reported, but a difference was found at the D-Oxz residue. The D-Oxz ring was turned over in relation to the disposition of the Oxz rings in natural ASC. |