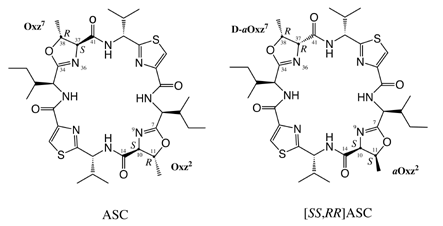
The natural ascidiacyclamide (ASC) has two Oxz residues having (S,R)-configurations. These chiralities are canged to (S,S) and (R,R). @Aug. 2002
| An ascidiacyclamide diastereomer (No.7) |

The natural ascidiacyclamide (ASC) has two Oxz residues having (S,R)-configurations. These chiralities are canged to (S,S) and (R,R). @Aug. 2002 |
 |
The peptide ring was very flat. Such a structure was the first example of ASC analogues.
Published: Akiko Asano, Takeshi Yamada, Atsushi Numata, Yoshio Katsuya, Masahiro Sasaki, Taizo Taniguchi, Mitsunobu Doi (2002) Biochem. Biophys. Res. Commun. 297, 413-417. A flat squared conformation of an ascidiacyclamide derivative caused by chiral modification of an oxazoline residue |
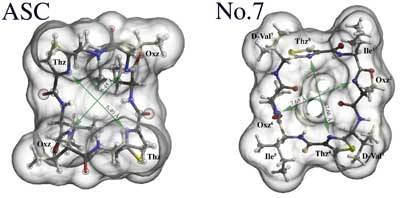 Drawn by Raster3D with water exclusive surface calculated by MSMS. Figure shows that No.7 has the cavity of the peptide ring, but ASC not. |
| formula | C36H52N8O6S2, 3(H2O), CH3OH | ||
| weight | 843.07 | ||
| symmetry | monoclinic | ||
| space group | P21 | ||
| Cell | Crystal | ||
| a | 14.4745(4) Ang. | description | block |
| b | 9.9580(2) Ang. | colour | Colorless |
| c | 15.3414(4) Ang. | size (mm) | 0.06x0.04x0.04 |
| alpha | 90 deg. | Dx (g/ml) | 1.271 |
| beta | 94.859(2) deg. | F(000) | 904 |
| gamma | 90 deg. | mu | 0.182 |
| volume | 2203.32(9) Ang^3 | wavelength (A) | 0.836 |
| Z | 2 | ||
| Diffrn measurement | Refinementspace | ||
| device type | Rigaku R-AXIS IV | Flack | 0.02(12) |
| Rint | parameters | 515 | |
| T | 100 K | restraints | 0 |
| theta max (deg.) | 30.0 | R_factor_gt | 0.536 |
| total reflections | 4543 | wR_factor_gt | 0.1349 |
| reflections(obs) | 4423 .gt. 2sigma(I) | dela rho_max | 0.555 e A^3 |
| Structure | space space space space space space | delta rho_min | -0.627 e A^3 |
| solution | SHELXS-97 | shift/su_max | lt.0.001 |
| refinement | SHELXL-97 | Goodness of fit | 1.136 |
